Well-prepared laboratories are first line of defence against novel coronavirus in Europe
WHO
Timely detection of a contagious pathogen is vital to controlling its spread. In response to the emergency of novel coronavirus (2019-nCoV), WHO is working closely with a network of designated laboratories in the WHO European Region and other WHO regions to ensure that every country has the capacity and resources required to quickly test, report and respond to any suspected cases of the disease. In particular, WHO/Europe is facilitating shipment of testing kits, within a month of the virus first being isolated.
Building a fortress of laboratories
Working largely unseen, in the context of routine disease surveillance or in the event of acute disease outbreaks, laboratories are a vital link in the chain of activities required to keep populations safe from diseases. Recognizing this, WHO/Europe and the European Centre for Disease Prevention and Control (ECDC) moved quickly following identification of the novel virus on 7 January 2020 to identify laboratory needs and capacities in the European Region.
WHO/Europe and partners established a roster of 6 international laboratories in the Region to act as regional referral laboratories to provide testing support to laboratories working at country level. These laboratories are located in France, Germany, the Netherlands, the Russian Federation and the United Kingdom. A network of nationally designated laboratories for testing of 2019-nCoV was also activated, primarily building on the existing laboratory network within the Global Influenza Surveillance and Response System (GISRS).
Testing of samples
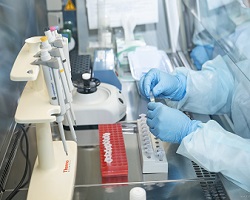
Thanks to China’s swift release of the full virus sequence, the first protocol for diagnostic testing of samples collected from potentially infected patients and their contacts became available in early January. This enabled 31 countries in the Region to rapidly establish testing capacities. WHO also commissioned the manufacturing of test kits for global distribution to the designated 2019-nCoV laboratories. WHO/Europe is facilitating a first round of shipment of 192 kits (containing 100 tests each) to 20 laboratories in the Region. Globally, WHO is making 250 000 tests available to 159 laboratories.
Countries with no testing capacity can send their samples to the WHO appointed 2019-nCoV referral laboratories for testing. New 2019-nCoV laboratories are also encouraged to send the first 5 positive and the first 10 negative 2019-nCoV samples to their referral laboratories for confirmation of test results.
To ensure rapid and free-of-charge shipment of samples, WHO repurposed the Shipping Fund Project established by the GISRS. In addition, WHO and partners are developing an external quality assurance programme for national 2019-nCoV laboratories.
This rapid expansion of testing capacity is increasing preparedness in each country to reduce the risk of infection and spread of the virus.
Status of spread in European Region
Available data on the spread of the virus changes daily. The vast majority of patients around the world is in China, and China’s strategy to contain the outbreak at its epicentre is proving effective, with only a few hundred cases so far in the rest of the world. This is the European Region’s window of opportunity to prepare in every country for a potential introduction of 2019-nCoV. This includes strengthening capacities to find, test and care for patients; to prevent and control infections; and to communicate risks with the public. For the latest situation report see the WHO website.



